For adults with moderately to severely active ulcerative colitis (UC) or Crohn’s disease (CD).
Learn from your peers
This Peer Perspectives resource center features ENTYVIO educational content, insights on clinical data from key opinion leaders, and materials related to the clinical efficacy of ENTYVIO for ulcerative colitis and Crohn’s disease.
GI Perspective
Get expert perspectives from leading gastroenterologists and listen to their thoughts about ENTYVIO. Learn more about clinical trial data, along with reviews of safety and efficacy for their adult patients on ENTYVIO.

ENTYVIO for UC
Aja McCutchen, MD
Dr. Aja McCutchen discusses the impact of the VARSITY trial, maintenance treatment options, and subgroup analyses based on prior treatment exposure and disease severity, and the pivotal GEMINI 1 trial.

Treating Early in Moderate to
Severe Crohn's Disease
Timothy Ritter, MD & Brooke
Hodnick, PA-C, MPAS
Dr. Timothy Ritter and his PA Ms.
Brooke Hodnick discuss early
intervention following the loss of
response to conventional therapy,
steroids, or TNF blockers for patients
with moderately to severely active
Crohn's disease.

Starting Advanced Therapy
Bincy Abraham, MD, MS
Dr. Bincy Abraham gives her
perspective on starting advanced
therapy, after failure with or loss of
response on conventional therapies
or steroids, with ENTYVIO.

ENTYVIO for Crohn’s
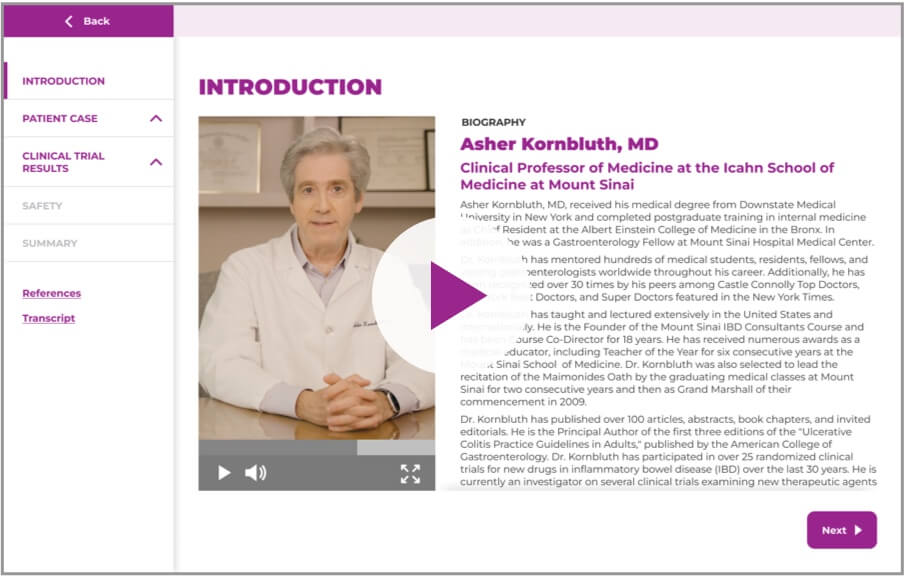
Asher Kornbluth, MD
Dr. Asher Kornbluth offers his
perspective on the most compelling
data from the GEMINI 2 trial.

The VARSITY Trial
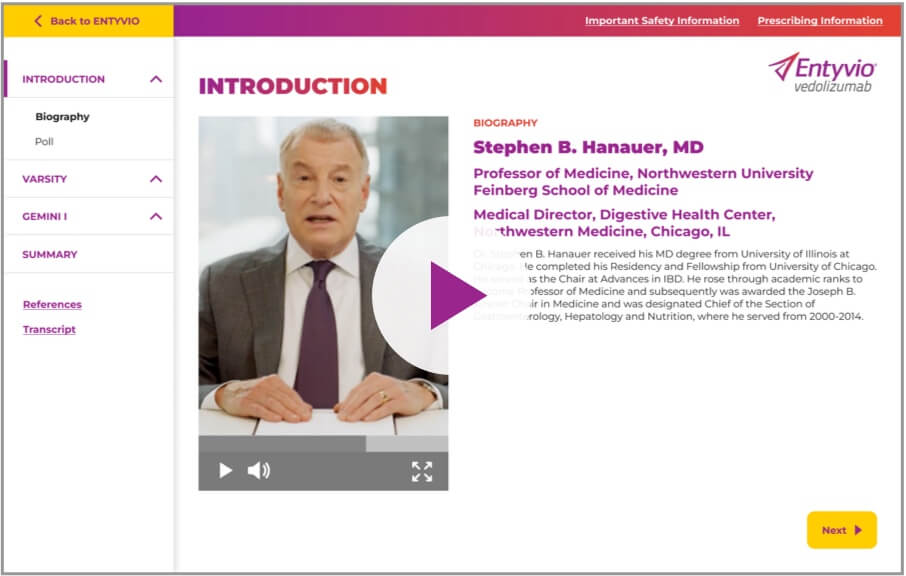
Stephen Hanauer, MD
Dr. Stephen Hanauer talks about the
importance of the head-to-head
VARSITY trial data in selecting a
treatment for ulcerative colitis.
Interactive Learning Modules
Explore interactive presentations from leading gastroenterologists on ENTYVIO—including head-to-head studies, appropriate patient types, and ENTYVIO's efficacy and safety profiles. Presenters are paid consultants for Takeda.
Clinical Reprints
Review insights on ENTYVIO clinical trial data for healthcare professionals.

VISIBLE 1 Trial Reprint
Efficacy and safety of vedolizumab
subcutaneous formulation in a
randomized trial of patients with
ulcerative colitis

VARSITY Trial Reprint
Vedolizumab versus adalimumab
for moderate to severe
ulcerative colitis

VARSITY Histologic End Points Reprint
Vedolizumab versus adalimumab
for moderate to severe
ulcerative colitis

COLOMBEL Trial Reprint
The safety of vedolizumab for
ulcerative colitis and
Crohn’s disease

VISIBLE 2 Trial Reprint
Efficacy and safety of vedolizumab
subcutaneous formulation in a
randomized trial of patients with
Crohn's disease
Explore more topics
Looking for patient support?
Want to talk to a Rep about
ENTYVIO?
The content on this page has been written and
reviewed by Takeda.